The
Dehydration of Alcohol:
Dehydration
of alcohol is an elimination reaction which is catalysed by acid.
In
this reaction, an alcohol is converted into alkene by loosing water in the
presence of acid and the application of heat.
The
reaction can be carried out in either of two ways.
·
By heating alcohol with sulfuric acid (H2SO4) or phosphoric acid (H3PO4)
·
By passing alcohol vapour over alumina (Al2O3)
which acts as an acid) at high temperature
Mechanism:
The
reaction takes place in three steps.
- Reaction between acid and alcohol gives the protonated alcohol and conjugate base of the acid.
- The protonated alcohol undergoes hydrolysis to form the carbocation and water.
- The carbocation looses a proton to the base to give alkene.
The
rate of dehydration depends upon last two steps; formation of carbocation and
loss of proton.
Ease of Dehydration:
The
various classes of alcohols differ widely in ease of dehydration. The order of
reactivity of alcohols towards dehydration is:
3⁰ > 2⁰
>1⁰
Tertiary
alcohols undergo dehydration the most rapily. This is because, they form the
most stable carbocations than any other alcohols and once these cations formed
they give the most stable alkenes.
Orientation of the reaction is strongly Saytzeff:
When there
is more than one type of β-hydrogens (β1 and β2) in alcohol then there is a
possibility of formation of more than one alkene. In such case, preferred
alkenes is more stable one, which can be identified by using Saytzeff’s rule. The
dehydration of alcohol is strongly oriented to saytzeff rule.
Saytzeff’s rule: According to this rule, the
preferred product is that alkene which is formed by removal of the hydrogen
from the β-carbon having the fewest hydrogen substitutents.
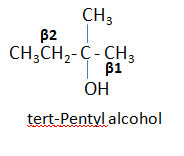
For example: In dehydration of tert-Pentyl
alcohol, two products 2-Methyl-2-butene and 2-Methyl-1-butene are formed.
‘Since there are two types of β carbon (β1 and β2), therefore
two alkenes are expected.
Here β2 is
having fewer number of hydrogen than β1, so according to Saytzeff’s rule
preferred product is formed by the removal of the hydrogen from β2. Thus 2-Methyl-2-butene
is obtained as main product.