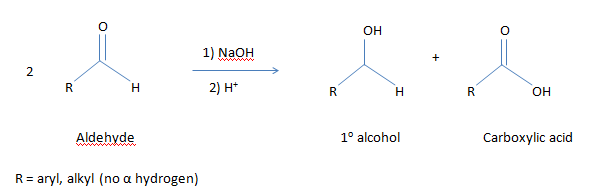
What is Cannizzaro Reaction?
The Cannizzaro
reaction, named after its discoverer Stanislao Cannizzaro.
In this reaction, two molecules of an
aldehyde are reacted with strong base to produce a primary alcohol and a
carboxylic acid using a hydroxide base.
- It is a redox reaction means in this reaction; the aldehyde undergoes both the oxidation and reduction. The oxidation product is a salt of a carboxylic acid and the reduction product is an alcohol.
- Only aldehydes that do not have alpha hydrogen show Cannizzaro reaction.(Alpha hydrogen is the hydrogen atom attached to C next to aldehyde functional group i.e. with (C* - CHO) like formaldehyde HCHO, acetaldehyde CH3CHO etc).
Mechanism:
The
reaction begins with hydroxide attack on the carbonyl carbon followed by
deprotonation to give a dianion.
This unstable intermediate releases a
hydride anion which attacks another molecule of aldehyde.
The alkoxide is more basic than water so it picks up a proton from water to gives alcohol as final product.