Carboxylic
Acid and its Acidity:
First
of all, what is carboxylic acid?
A
compound which contains the carboxyl group (-COOH) attached to hydrogen (H –
COOH), an alkyl group (R – COOH) or an aryl group (Ar – COOH) is called
carboxylic acid.
For
Example:
Why does it
call an acid?
The functional group of carboxylic acid is
carboxyl group i.e. –COOH. The carboxyl group (-COOH) is a combination of two
groups; carbonyl group (>C=O) and hydroxyl group (-OH). The –OH of a carboxylic acid tends to release
H ion.
If we consider the ionization of carboxylic
acid, we find that
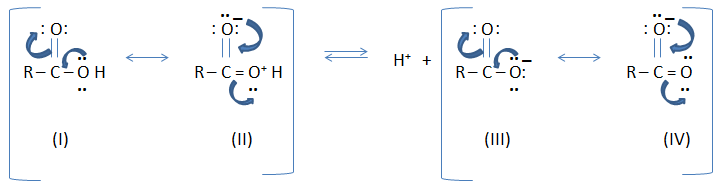
Carboxylic acid is a resonance hybrid of two
non-equivalent structure (I and II) involving separation of charges while
carboxylate anion is a resonance hybrid of two equivalent structures (III and
IV).
As a result, carboxylate anion is far more stable than carboxylic acid.
The equilibrium of the reaction is shifted towards increase ionization. (It
means a carboxylic acid tends to release H ion and forms a carboxylate anion) Thus,
carboxylic acids are acidic in nature.
Carboxylic
acid is more acidic than phenol and an alcohol:
If we compare an alcohol, phenol and a
carboxylic acid, we find that from acidity point of view they are:
Carboxylic acid > phenol > alcohol
All these compounds have same functional group
i.e. –OH; but carboxylic acid is most acidic in them. It means the –OH of a
carboxylic acid loses a hydrogen atom more readily than the –OH of an alcohol
or a phenol.
To understand it better, let us consider ionization
of alcohol, phenol and carboxylic acid.
Now
first let us compare the acidity of phenol and alcohol.
Due
to delocalization of the negative charge over the ortho and para positions of
aromatic ring, phenoxide anion is more stable than phenol. This favours the
ionization of phenol.
However,
if we observe the ionization of alcohol, alcohol and alkoxide anion are each
represented by a single structure. In an alkoxide anion the negative charge is
localized on a single oxygen atom. Thus, phenols are acidic than alcohols.
Let
us compare the acidity of carboxylic acids and phenols.
Carboxylate
ion has two equivalent resonance structures in which the negative charge is
delocalized over the more electronegative two oxygen atoms.
Phenoxide anion has
two non-equivalent resonace structures in which the negative charge is
delocalized over one oxygen atom and less electronegative carbon atom.
Consequently carboxylate anion is more stable than phenoxide anion. Thus
carboxylic acid is more acidic then phenol and alcohols.
Effect of
Substituents on Acidity:
When
a carboxylic acid bears any substituent, its acidity affects. So now let us see
how changes in the structure of the carboxylic acid affect the acidity.
We
know that ionization of carboxylic acid in an equilibrium process.
- Any substituent that stabilizes negavtively charged carboxylate
anion more than it stabilizes the acid should increase the acidity.
Electron-withdrawing substitutents (-Cl, -CN, -NO2) increase the acidity of
carboxylic acid by dispersing the negative charge by inductive effect and
stabilizing the carboxylate anion.
- On the other hand, any substituent that makes the anion less
stable should decrease acidity. Electron releasing group (-CH3, -OH, -OCH3,
-NH2) decreases the acidity of carboxylic acid by intensifying the negative
charge(by inductive effect) and destabilizing the carboxylate anion.
The
inductive effect depends upon number of substituents and the electro
negativity. So….
- In aliphatic carboxylic acid, as the number of substituent i.e. electron
withdrawing group increases, the acidity of the acid also increases.
- As the electron withdrawing
substituent moves farther from the carboxyl group, the strength of the acidity
decreases.
(This
is because; as the distance increases, electro-negativety decreases and this results
decrease in inductive effect)
- It means substituents on α carbon atom are most effective in
increasing the strength of the acid.
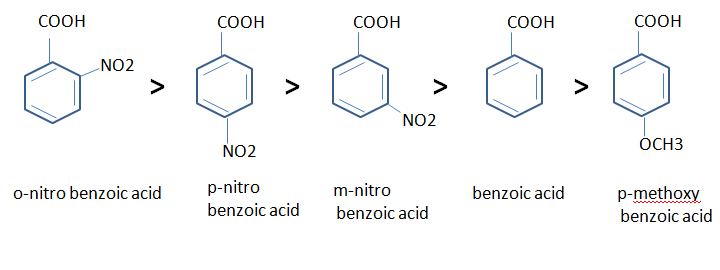
- In aromatic acid too, electron withdrawing groups increase the
acidity whereas electron releasing groups decrease the acidity.
- Direct attachment of phenyl or vinyl group increase the acidity of
corresponding acids. This is due to the greater electro negativeity of the sp2
hybridised carbon atom to which the carboxyl group is attached. The inductive effect
of the following groups in the decreasing order of acidity is
- The –OH and –OCH3, groups display both kind of effect if they are
attached to para or meta position of an aromatic acid.
- If they are
attached to meta position, they show an electron-withdrawing acid-strengthening
inductive effect.
- If they are
attached to para position, they show an
electron releasing acid-weakening resonance effect.